Qvin's world-first diagnostic menstrual pad gets FDA clearance
Initially cleared for diabetes, the Q-Pad's removable collection strip makes blood sugar testing easy and pain-free
A world-first period pad that can be used for diagnostic tests has received FDA-clearance today (9 January 2024).
The Q-Pad by biotech company Qvin collects menstrual blood samples to be sent off for testing, as an alternative to traditional and invasive blood tests.
While initial FDA-clearance is for diabetes glucose monitoring, the clearance brings closer many more opportunities for using menstrual blood as a diagnostic and for monitoring.
Co-founder, Medical doctor and scientist Dr Sara Naseri said:
“Menstrual blood - the most overlooked opportunity in women’s health, a waste product, and in many cultures still a stigma and taboo - is as of today no longer just waste but in fact a valuable, accessible and non-invasive source of health information.”
"With the first ever FDA-cleared menstrual blood health test, Qvin is paving the way to important new opportunities for women's health and this is just the beginning,"
"We are simplifying routine testing, and freeing up resources that can be used on providing care and ultimately our goal is to make health care much more accessible."
How Qvin uses menstrual blood
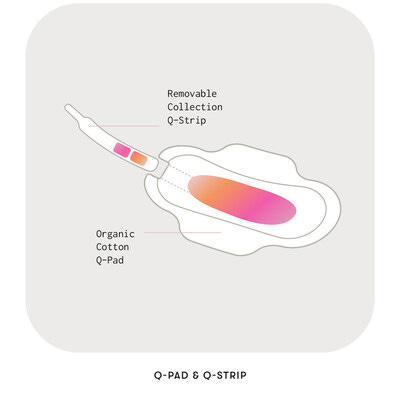
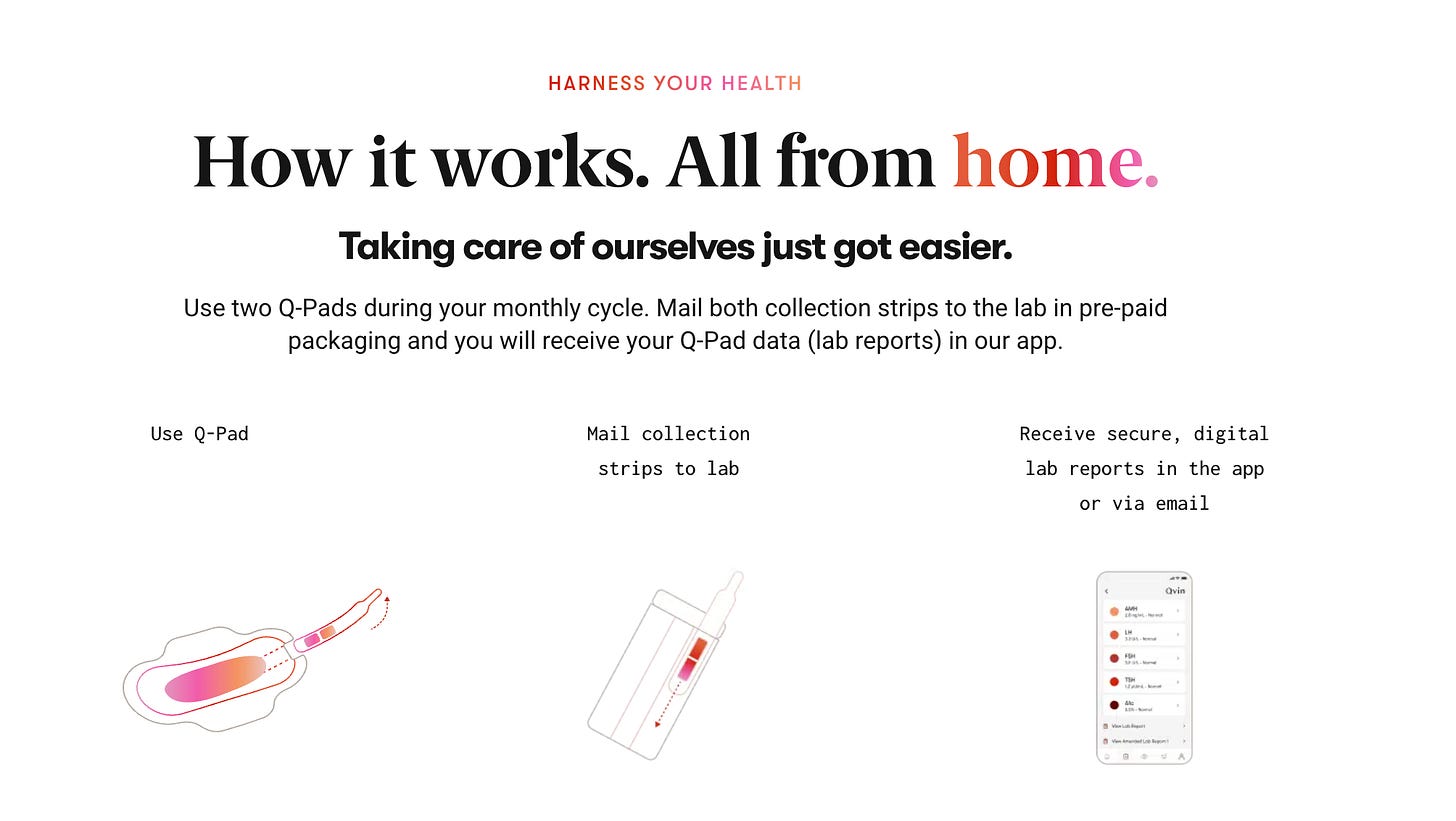
The Q-Pad is easy to use. It has a tear-off collection strip which is torn off after the pad is used. The instruction is to use two Q-Pads during each monthly cycle and then to mail both collection strips to the lab in pre-paid packaging.
Users also receive their lab reports in an app and /or over email.
The first clearance is specific to the A1c - a routine test used to monitor blood sugars for diabetes and pre-diabetes. Trials have shown the testing of menstrual blood using the Q-Pad is as precise as a normal blood test taken in a doctor’s office.
Sara said:
“The Q-Pad is so novel that the FDA had to create an entirely new product category for us which, appropriately, has a code starting with ‘Q.’”
The possibilities of periods
Menstrual blood has been ignored as an opportunity in women’s health. Yet Qvin has been exploring its potential for over a decade.
Sara said:
“It was [in 2014] that I and the Qvin team set out to prove that menstrual blood is not waste, but a medical wonder, refusing to take shortcuts as we did. Tens of thousands of hours spent on trials, thousands of pages of novel research, and many peer reviewed papers later, we’ve now passed the highest bar that exists for medical products by receiving the FDA’s stamp of approval.
”All those years ago, we believed that menstrual blood could be as powerful for diagnostics as normal blood. The data, the science and the regulators have now all weighed in: it is.”
Qvin is now working to make this technology more accessible. In collaboration with researchers at academic institutions such as Stanford University School of Medicine, Qvin has published peer-reviewed research validating other biomarkers that can also be monitored.
The Q-Pad allows individuals to submit specimens directly to the lab and receive reports on key health conditions that often go undiagnosed or misdiagnosed including:
pre/diabetes
anemia
fertility
perimenopause
endometriosis
thyroid health.
Co-founder Søren Therkelsen said:
"Utilizing menstrual samples, the Q-Pad can address critical women's health issues that have historically been neglected."
.