Teal Health awarded $1.68m grant for at-home cervical cancer screening
The Small Business Innovation Research (SBIR) grant cam from the National Cancer Institute (NCI) of the National Institutes of Health (NIH)
Teal Health, a woman-led company on a mission to get all women and people with a cervix in the US screened for cervical cancer, has been awarded a $1.68 million Small Business Innovation Research (SBIR) Direct to Phase II Grant from the National Cancer Institute (NCI) of the National Institutes of Health (NIH).
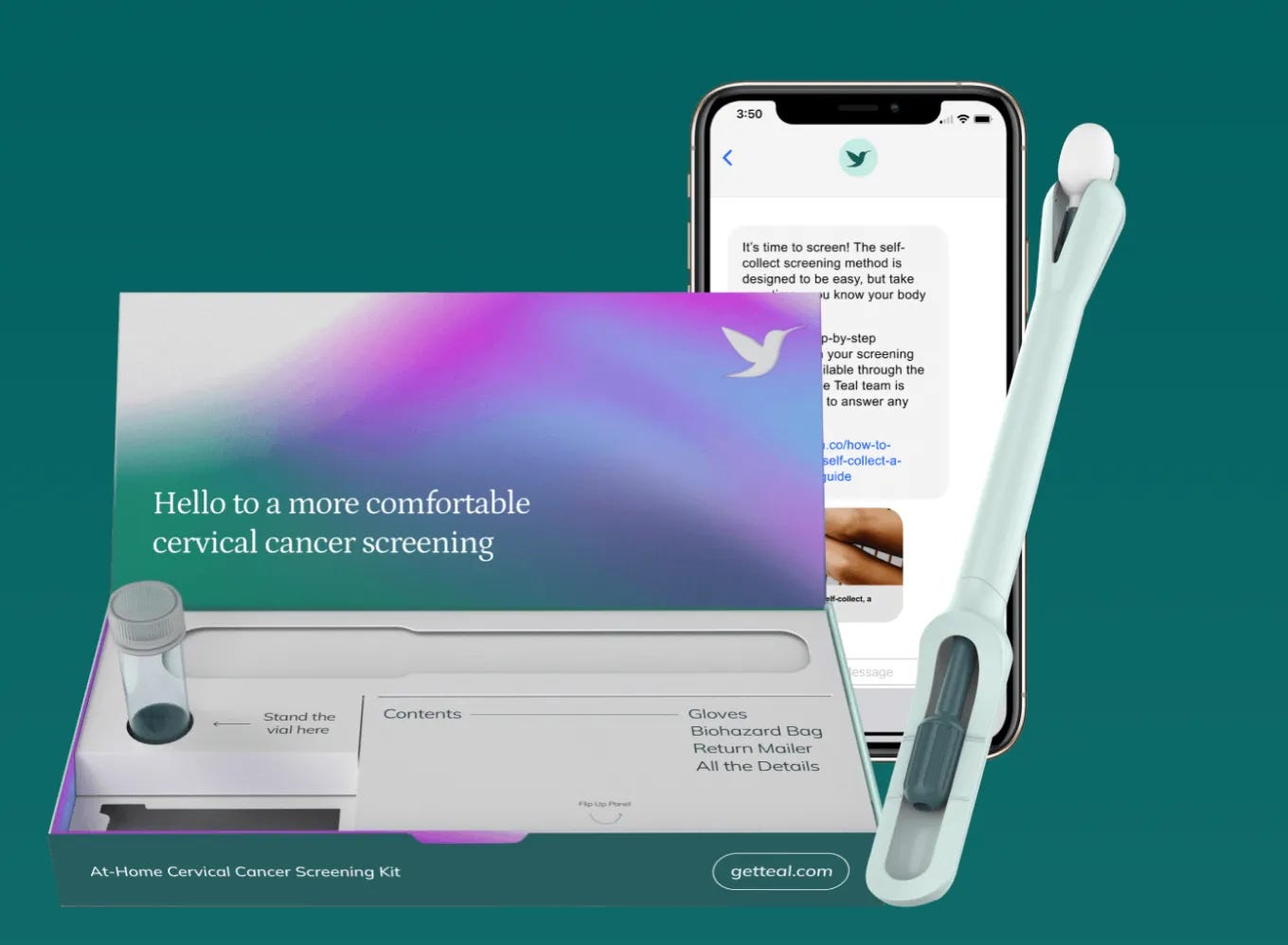
The prestigious grant was awarded to support the development and clinical validation of Teal Health's Teal Wand, a purpose-built device that allows a woman to collect her own vaginal sample for human papillomavirus (HPV) testing, a critical piece in cervical cancer prevention - all from the comfort, convenience, and privacy of her home.
Significant need
The grant follows a highly competitive review process, with only a limited number of applicants selected for funding in this review period.
Teal Health shared that the NIH committee recognised the importance of the Teal Wand, coupled with the telehealth technology platform, to increase access to cervical cancer screening and adherence to follow-up steps.
Teal Health was chosen for its potential to address a significant need in women's health: increasing access to life-saving cervical cancer screening, of which 1 in 3 women are not up-to-date.
Kara Egan, CEO and co-founder of Teal Health, said:
"We're honoured that the NIH recognises the importance of our at-home screening technology in increasing access to preventive care and cancer screenings for women in the US.”
"This funding brings us closer to our goal of providing women greater autonomy over their health while advancing the fight to eradicate cervical cancer."
Supporting clinical development
The SBIR funds will support the clinical development of the Teal Wand, for which a nationwide clinical trial was launched in early 2024. Clinical validation is essential to providing women with a trusted and accurate alternative to in-office testing, and it is equally important that the women who use it feel confident and comfortable, and find it easy to use. In May, Teal Health was also granted ‘Breakthrough Device status’ by the US Food and Drug Administration (FDA).
Teal Health has partnered with leading institutions and experts, including Dr Akiva Novetsky, Associate Chair for Quality and Safety at New York Medical College and Associate Professor of Gynecologic Oncology at Westchester Medical Center.
Dr Akiva Novetsky said:
"As we work towards the elimination of cervical cancer in the US, this grant will help provide safety and efficacy data for at-home self-collection, an option that has the potential to further expand access to cervical screening."
"This level of clinical rigor is essential to ensure that this screening method has similar efficacy to clinician-collected screening. We are hopeful that self-screening will help in closing the screening gap while maintaining the highest standards of care."