Ultrahuman partners with Click Therapeutics on biomarker-driven migraine tool
Women experience migraine at nearly three times the rate of men
A new partnership between wearable health company Ultrahuman and prescription digital therapeutics firm Click Therapeutics aims to bring migraine management out of the clinic and into everyday life, using biometric data and software rooted in FDA-authorised technology.
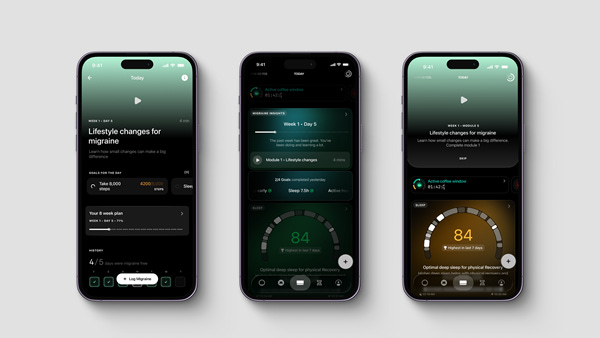
The collaboration sees the launch of Migraine PowerPlug, a software feature within the Ultrahuman platform that draws on the proprietary technology behind Click’s digital migraine therapeutic. The tool is expected to roll out to users in early 2026 following a pilot phase, with availability planned across the US, Canada, Europe, India and Australia.
The companies describe Migraine PowerPlug as the first biomarker-driven migraine management tool of its kind built on FDA-authorised digital therapeutic foundations. While it will not itself be a prescription treatment, the feature is designed to translate wearable data into personalised insights and guided behavioural activities for people living with migraine.
“Helping people improve how they feel daily is core to Ultrahuman’s mission. By integrating the underlying principles of Click Therapeutics’ digital migraine treatment technology into our latest PowerPlug, we are enabling users to track and understand migraine-related patterns in real time, and then go further to turn those insights into actionable guidance,” said Mohit Kumar, CEO of Ultrahuman.
A widespread and gendered burden
Migraine affects more than one billion people globally and remains one of the most disabling neurological conditions worldwide. Women bear a disproportionate share of that burden, experiencing migraine at nearly three times the rate of men. It is also the leading cause of disability among women aged 15 to 49, disrupting work, family life and overall wellbeing.
Despite its prevalence, migraine care is often fragmented. Clinical appointments are intermittent, while symptoms and triggers unfold daily. Many patients are left to self-manage patterns linked to sleep, stress, hormonal change and activity with limited support between visits.
The Ultrahuman–Click partnership is positioned as an attempt to bridge that gap by embedding migraine-specific guidance into a consumer wearable ecosystem that users already engage with throughout the day.
From tracking to intervention
At the core of Migraine PowerPlug is the integration of Ultrahuman’s biometric analytics with the principles behind Click’s FDA-authorised digital therapeutic, CT-132. That prescription product is designed to support the preventive treatment of migraine when used alongside medication, using a clinically validated behavioural approach to reduce migraine burden.
Rather than offering passive tracking alone, the new PowerPlug is being developed around digital therapeutic design principles. Users will be able to observe trends across sleep, heart rate variability (HRV), stress and movement, and receive tailored lifestyle guidance intended to help manage potential triggers.
Planned features include goals for daily activity, sleep consistency and personalised hydration recommendations, based on an individual’s observed migraine patterns. The aim, the companies say, is to help users identify correlations in their own data and apply evidence-based strategies to build resilience over time. They stress however that the PowerPlug itself is not a prescription digital therapeutic.
“Our partnership with Ultrahuman will allow us to bring a much-needed personalized migraine solution to people around the world,” said David Benshoof Klein, CEO and founder of Click Therapeutics.
“As digital health evolves, combining our clinically validated digital treatments with personalized consumer biometrics allows us to address an unmet need in over-the-counter care and the fast-growing wearables market.”
Migraine, women’s health and wearables
Ultrahuman has increasingly positioned women’s health as a strategic focus, including through its recent acquisition of viO and its OvuSense ovulation tracking technology. For many women, hormonal fluctuations are closely linked to migraine frequency and severity, making continuous physiological monitoring particularly relevant.
By layering migraine-specific insights onto cycle, sleep and recovery data, the company argues that users may gain a clearer picture of how hormonal and lifestyle factors interact, potentially informing earlier or more proactive interventions.
Earlier this month another leading wearable, Oura ring, announced a partnership with Aptar Digital Health to integrate Aptar’s Migraine Buddy tracking app into the Oura ring.



So interesting, one of my closest friends suffers from daily chronic migraines, she will be so pleased to hear about this!