Visby Medical raises $55m to launch at-home STI testing to women across the US
Funding may reach $65m in total says Visby Medical
Visby Medical, a California-based diagnostics company, has raised $55 million in a new financing round to accelerate the rollout of its recently FDA-authorised at-home sexually transmitted infection (STI) test for women.
The round, led by Catalio Capital Management, includes participation from ND Capital, Cedars Sinai Medical Center, Blue Water Life Science Advisors, Pitango Ventures, and long-time health investor John Doerr.
The funding may reach $65 million in total and will support manufacturing scale-up and distribution efforts ahead of the product's launch in July 2025.
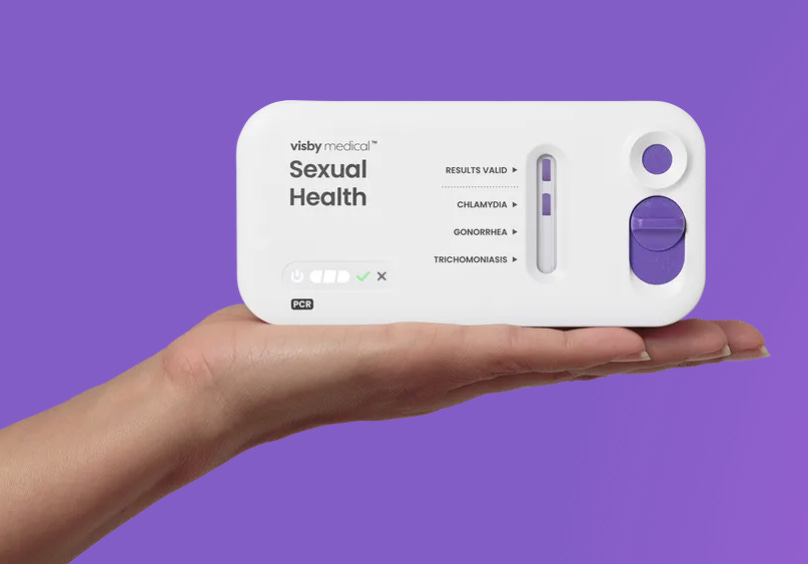
Visby’s Women’s Sexual Health Test is the first single-use, disposable PCR diagnostic device designed for at-home use. The test delivers laboratory-grade results for common STIs in under 30 minutes via a smartphone app, and connects users to telemedicine providers for follow-up care — offering a discreet, fast, and clinically accurate alternative to in-clinic testing.
“We are excited to partner with Catalio Capital Management, a true industry powerhouse, as we advance our mission to transform healthcare through rapid and convenient at-home diagnostics that deliver the same accuracy as traditional PCR machines,” said Adam de la Zerda, PhD, Founder and CEO of Visby Medical.
“This funding round will enable Visby to deliver on our vision of empowering consumers with reliable and lab-accurate health information from the comfort of their homes, starting with our at-home test for sexually transmitted infections for women.”
STIs continue to be undiagnosed
The innovation comes at a critical time: STIs remain highly prevalent across the United States, disproportionately affecting women, and often go undiagnosed due to stigma, lack of access, or long clinic wait times. While home testing options have expanded in recent years, most rely on mail-in samples — delaying results and treatment. Visby’s offering aims to remove those barriers, especially for women who may otherwise avoid testing.
“Visby Medical has delivered the first and only laboratory-grade STI testing solution that can be made directly accessible to individuals,” said Catalio partner Isaac Ro.
“We were eager to invest in Visby ahead of their FDA clearance, knowing this would represent a breakthrough moment for the diagnostics industry.”
Alongside the financing, Visby also announced the appointment of two new board members: Isaac Ro, who joins as a board observer, and Chuck Alpuche, former COO of Insulet Corporation and a veteran in operational scale-up and manufacturing.
Alpuche said: “Visby's FDA-authorized at-home STI test is redefining convenience and accessibility in diagnostics... I'm particularly excited to contribute my operational experience to help Visby streamline manufacturing and drive down unit costs — essential steps in making the technology accessible to millions.”
The company has stated its intent to expand beyond STI diagnostics into other at-home testing categories, but for now, its women-focused sexual health test stands as a key step toward closing gaps in access to sexual healthcare — combining speed, privacy, and accuracy in a handheld device.