💌 Issue 122: Women's health gets cancelled | YON E Health raises €250K | Endometriosis test prepares for first launch | FDA blackbox warning removal
The global weekly briefing on women's health innovation and FemTech
Welcome to issue #122 of FutureFemHealth, (w/c November 10 2025) — the global weekly briefing on women’s health innovation, trusted by 8,700 investors, innovators and leaders to decode the funding flows, breakthrough ideas and policy shifts transforming the sector.
🌟 In this week’s briefing:
🤐 Women’s health is still getting censored
✅ YON E Health raises €250K to bring data-driven insight to vaginal health
❤️ Endometriosis test EndoID prepares for first launch
🔥 FDA approves removal of blackbox warning from menopause hormone therapy
Got news to share from the world of FemTech and women’s health innovation? Let me know at anna@futurefemhealth.com
This week’s newsletter is powered by Daya Ventures….
We’ve talked about the gender health gap for years - but if the same hands hold the equity, the system will not change.
Daya Ventures did it differently: in two years they built 18 women’s-health startups, 98% majority-owned by women. Their community round hit its first goal in six hours and is now backed by a sharp mix of first-time women investors, veteran angels, and respected tech/impact leaders ready to build—and own—the future of women’s health.
Now, there’s still time for you to get involved*…
*Capital at risk.
🤐 “This should be illegal”
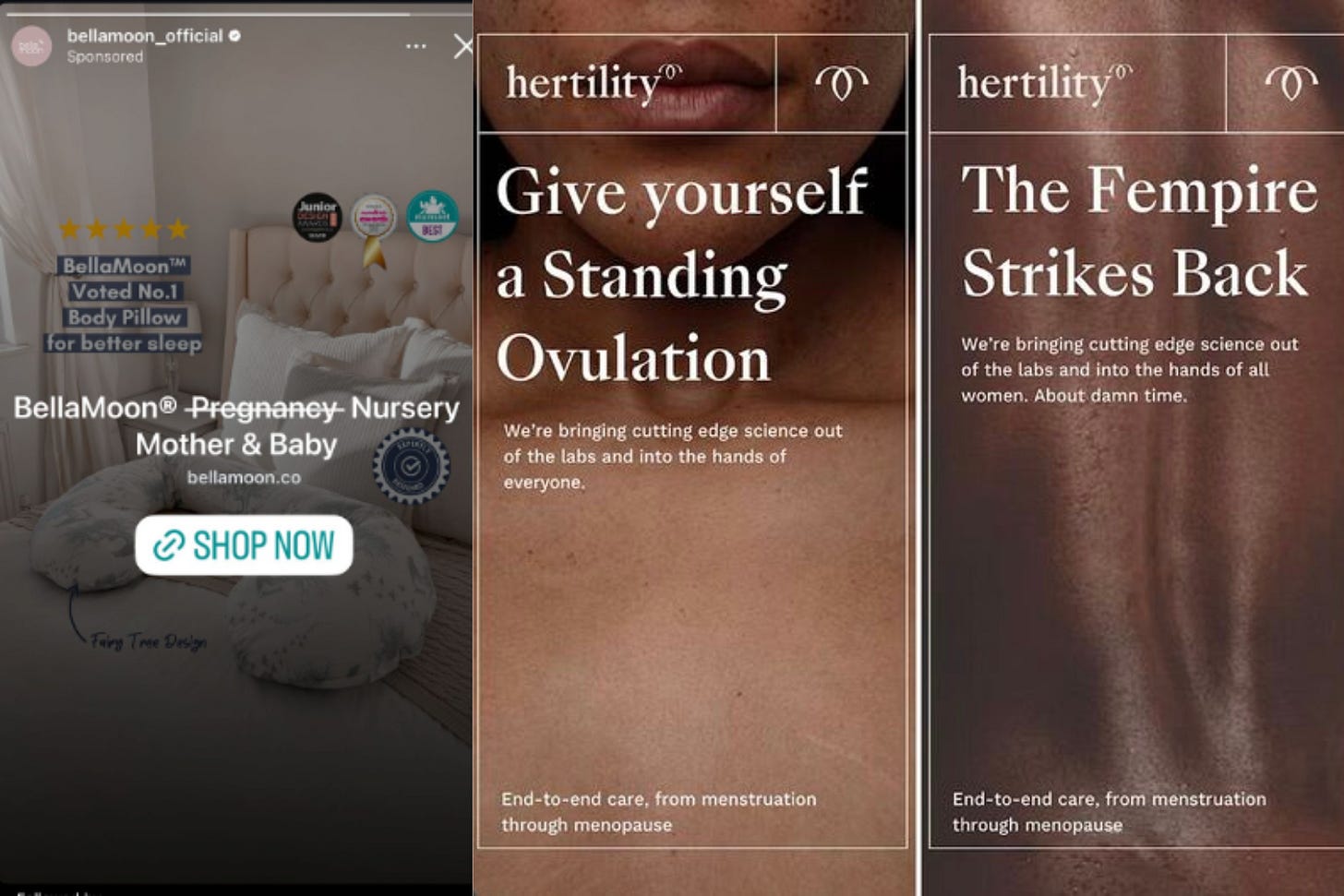
In case you were in any doubts…censorship of women’s health content has not gone away.
Hertilty’s ads for its hormone test have been completely banned by Meta for most of 2025 - the company’s website traffic has dropped by up to 600,000 visitors a month. All the while men’s hormone ads have continued to run.
Then there’s BellaMoon. Its ads for a pregnancy pillow were classified as a medical device; Meta even crossed out the word pregnancy in its ad copy. After spending more than £100,000 in wasted ad spend this year alone, founder Irene Breen says she’s had to rename her own product a “three-in-one pillow” just to get it through the system.
As part of CensHERship, my co-founder Clio Wood and I have been supporting both Hertility and BellaMoon since the summer — helping to collate evidence, file complaints under the Digital Services Act and with Ofcom, and, most recently, supporting them to share their stories with the media.
This weekend the story reached a national audience through The Independent:
Getting such strong case studies placed in a major outlet and seeing Meta, Google and TikTok all acknowledge the issue publicly marks real progress. Those same press offices usually decline to engage at all.
From progress to the absurd
But even as this story made headlines, a twist none of us saw coming…
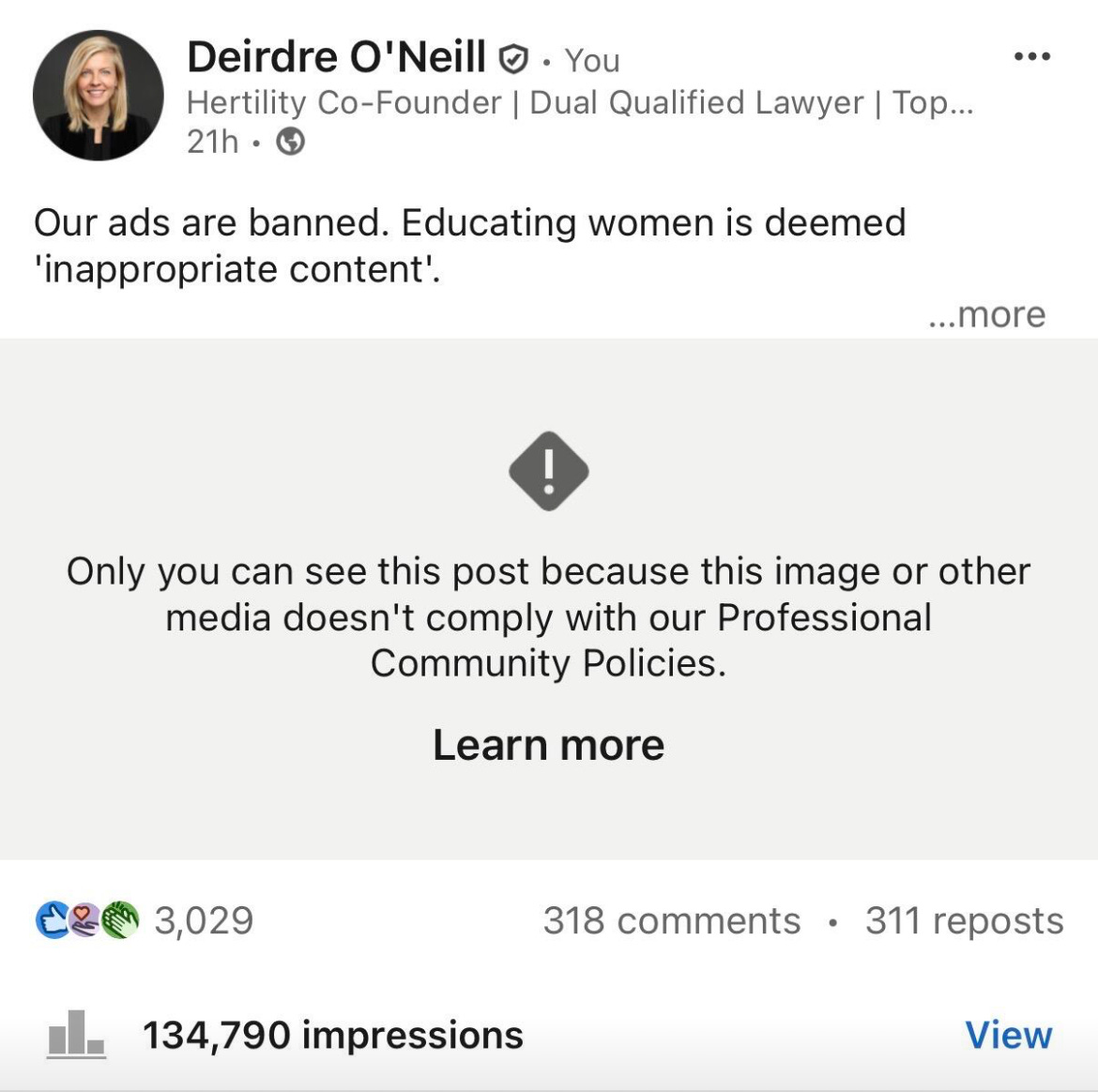
Yesterday, Hertility’s co-founder Deirdre O’Neill shared the article on LinkedIn. Her post gained more than 3,000 likes and hundreds of shares.
And then, LinkedIn deleted it.
Yep — a post about censorship, censored itself. You couldn’t make it up.
While the post was eventually reinstated, it’s another example of how censorship hasn’t gone away - it’s evolving.
In our research prior to this summer, LinkedIn was hardly ever referenced as a problem; and Meta’s new ‘health policy’ which triggered the bans above only came in this January/February. It’s getting more complex, more opaque, more frightening - and it needs more of us speaking out.
Credit to voices like Dr Aziza Sesay, Cindy Gallop, Jane Evans, the teams at Evofem, Center for Intimacy Justice, Frida, Daye, HANX and our partners The Case For Her. There are many more so apologies for any exclusions!
As for CensHERship, next week, we join another strong ally, global women’s-health brand Essity, for a parliamentary roundtable sponsored by Emily Darlington MP.
Now it’s time to turn evidence into action. While in the EU we’ve submitted nine complaints so far under the Digital Services Act, in the UK there is still no strong regulatory lever to challenge online censorship. The Online Safety Act aims to prevent harm — but not to protect fairness.
If the women’s health industry is to reach its potential and contribute an estimated $1 trillion annually by 2040 - and make a difference to billions of lives worldwide - it can’t be blocked at the first hurdle: basic online communication.
Get involved:
👩🏽💻 Share your own examples of censorship in our short survey - because building collective evidence is the most powerful way we can bring about change.
📄 Download our white paper ‘Censorship Revealed’ for the full story on censorship.
This week’s poll
💰 Capital flows: where are investors placing bets?
📌 THE NETHERLANDS: YON E Health raises €250K to bring data-driven insight to vaginal health. When founder Roswith Verwer began her company she was told vaginal health was too taboo, too complex and too ‘niche’ for innovation and investment. Now, addressing the ‘guessing gap’ when it comes to vaginal wellbeing, Roswitha is bringing her vision to life with her smart device that measures vaginal pH and basal body temperature - two biomarkers critical for fertility tracking, infection detection and hormonal health. This pre-seed round was led by PMK Group and will allow YON E Health to prepare for medical trials. (Continue reading: FutureFemHealth)
📌 U.S: Organon divests Jada System in $465m deal. One hundred employees working on the postpartum uterine bleeding system will transfer to medtech innovator Laborie Medical Technologies. Organon had previously taken over Jada System in 2021 as part of a buyout of Alydia Health. Organon’s interim CEO Joseph Morrissey said Laborie was well-postioned to further expand Jada and that the divestment was “another step towards improving capacity in Organon’s balance sheet to be able to pursue other growth opportunities in women’s health biopharma in the future.” (Continue reading: Fierce Biotech)
📌 SWEDEN: From innovation to ownership: inside Daya Ventures’ Community Round. Having already smashed its initial $250,000 goal within six hours, venture studio Daya Ventures is now in overfunding on Seedrs. The round brings 178 investors — from first-time women investors to seasoned angels — onto the same cap table. With 18 women’s-health ventures built to date (98% women-owned), Daya is reimagining not just who innovates in women’s health, but who owns it. (Continue reading: FutureFemHealth (Paid partnership. Capital at risk))
🌟 Industry moves and strategic shifts
📌 CANADA: SYNG Pharma prepares for first launch of endometriosis test EndoID. Endometriosis notoriously takes up to a decade to diagnose. SYNG Pharma wants to change that. Its dual-biomarker test EndoID, aims to triage patients early by offering a first-line, at-home screening before invasive procedures are needed. Fresh off regulatory approval, the company plans its first launch in India in early 2026, with UK and EU rollouts to follow after CE marking. “For a 14, 15-year-old young girl who would not go for invasive procedure… we wanted to have an accessible and affordable solution,” says founder Vinay Singh. (Continue reading: FutureFemHealth)
U.S: This wearable detects perimenopause: IdentifyHer to launch Peri. Perimenopause is one of the most common yet least understood health transitions women face. IdentifyHer, co-founded by Heidi Davis and Dr. Donal O’Gorman, wants to change that with Peri, a new wearable that uses biosensing and AI to track patterns linked to hot flashes, anxiety, sleep issues, and mood shifts. Billed as the first device built specifically for perimenopause, Peri turns vague symptoms into actionable data — helping women validate their experiences, share insights with clinicians, and make informed choices. Priced at $449 it won’t be accessible to all, but it’s HSA/FSA eligible and has already earned a CES Innovation Award winner. Beyond the device, a bigger play too is helping to close the data gap on how perimenopause affects long-term health. (Continue reading: FutureFemHealth)
📌 U.S: Women’s health app Rosy to close. Known for its trusted answers to health questions and a particular focus on sexual wellness, Rosy will close on November 20 after seven years. “Most importantly, Rosy has helped give women the language, confidence, and permission to talk about their sexual health without shame or embarrassment” wrote founder Lyndsey Harper and team on Instagram. (Continue reading: Rosy Wellness)
📌 UK: Period poverty charity Bloody Good Period announces closure. Founded in 2016, Bloody Good Period supported marginalised communities with access to menstrual supplies and worked to address the wider stigmas and shame that surround the topics of periods. It says it has now been forced to close, in part due to the severe financial pressures facing the wider charity sector. Founder Gabby Jahanshahi-Edlin, who stepped down back in 2022, said the closure was “devastating for those who relied on the services.” (Continue reading: Third Sector)
📌 CANADA: How doctor-founded Elan Healthcare treats the root cause with prevention-first women’s health supplements. After years in both clinical practice and pharma, Dr. Pari Saharkhiz grew frustrated that medicine waited for illness instead of anticipating it. With up to 70% of PCOS cases going undiagnosed until fertility struggles appear, her company Elan Healthcare is betting on targeted, natural supplements to support issues earlier. The aim: bring lifestyle and prevention into the same conversation as treatment. (Continue reading: FutureFemHealth)
🩸 Research and women’s health news
📌 U.S: FDA drops safety warnings on menopause hormone therapies. A landmark decision this week, as the FDA announced it will finally remove the prominent ‘black box’ warning from menopause hormone therapies, acknowledging that new evidence shows lower risk than previously believed. The move follows tireless campaigning, an expert panel hearing in July and a public comment period. Updated product labeling will now no longer include references to elevated “risks of cardiovascular disease, breast cancer, and probable dementia.” However, the agency will retain the boxed warning for endometrial cancer in estrogen-only products. (Continue reading: CNN)
📌 DENMARK: 97 % of Danish women report menopausal symptoms. A new survey from the University of Copenhagen reveals that a staggering 97% of women aged 45–59 in Denmark experience symptoms during the menopausal transition. While that data may not be entirely surprising, the sheer scale of the survey drives home the point - with 153,800 respondents it makes the survey one of the largest of its kind. Among them, 83% report at least one symptom of moderate to severe intensity, and 28% say they’re battling six or more symptoms. The standout complaints: sleep disturbances (54%), hot flushes (43%), exhaustion (39%), joint/muscle issues (39%) and sexual problems (35%). The researchers say these numbers show menopause isn’t a rare or mild phase — it’s a serious public-health challenge that deserves better attention and support. (Continue reading: University of Copenhagen)
📄 Policy watch: risks and opportunities
📌 EUROPE: ‘My Voice, My Choice’: MEPs support citizens’ initiative on accessible abortion. EU lawmakers have backed a citizens’ drive for safe, accessible abortion — urging Brussels to fund a mechanism that lets member states offer care to women denied it at home. The vote is perhaps a surprising win for reproductive rights given the wider political landscape in the world and Europe’s own divisive approaches to the issue. (Continue reading: European Sting)
📆 Save the date
DENMARK: Connecting the women’s health ecosystem: Copenhagen, Tuesday Nov 25, 5.30pm CET.
I’m co-hosting a networking meet-up with the team from Women’s Health Horizons and the Nordic Women’s Health Hub in Copenhagen later this month. We’ll have a short panel focused on connecting the women’s health ecosystem globally, with plenty of time for refreshments, networking, and new connections. If you’re working in women’s health as a founder, researcher, academic, investor, government or policy expert we’d love to see you!
UK: How to advocate for yourself. Virtual, Tuesday 18 November, 5pm GMT.
If navigating your own health has felt anything but straightforward, you’re not alone. Co-hosted by the healthtech startup Nexus, and featuring Dr Sula Windgassen, this panel will share personal and professional stories and includes a group workshop too.
That’s all for this week! If you’ve missed any previous newsletter issues catch them all at futurefemhealth.com and do make sure to follow us on LinkedIn.
Anna
PS. FutureFemHealth reaches 8,700 decision-makers and professionals in women’s health each week - from investors and founders to healthcare leaders and corporates. To explore partnership opportunities or request our media pack contact: anna@futurefemhealth.com








On your comment about Deidre O'Neill's LinkedIn post being censored by LinkedIn, it is now visible again😅.
Can you recommend any women’s health Subtack’s to follow! I am new here. Great newsletter thank you.