💌 Issue 107: $55m for at-home STI testing | Maven ŌURA partnership | approval for saliva-based contraceptive in Europe
+ lots more in your weekly round-up of women's health innovation and FemTech news
Hello and welcome to issue #107 of FutureFemHealth (w/c June 23 2025).
🌟 Coming up today we’ve got:
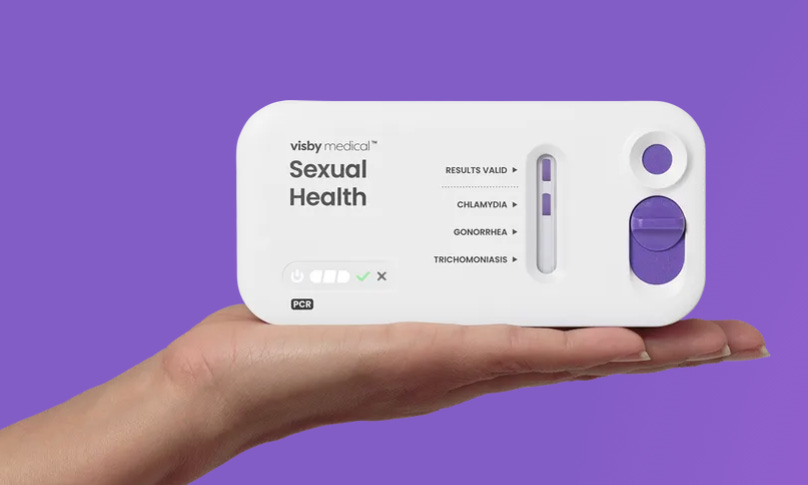
💰 Visby Medical raises $55m to launch at-home STI testing to women across the US.
📈 Maven Clinic and ŌURA partner to deliver more connected experience for women's health.
✅ Inne's saliva-based contraceptive secures European regulatory approval.
❤️ Women in England who don’t come forward for cervical cancer screening to be offered home tests.
Got news to share with our 8,300 global readers who work in women’s health and FemTech? Or would you like to sponsor the newsletter and reach our audience? Reply to this email to find out more or drop me a note at anna@futurefemhealth.com
The non-hormonal contraceptive worth waiting for?
Rewind to September 2016. Hormonal birth control dominates and fertility awareness is considered niche.
But founder Eirini Rapti was already working on something new: a hormone-free contraceptive that tracks hormone patterns through spit, using a small, at-home device.
This week, after nearly nine years of research, iteration and regulatory approvals, Inne is finally certified for use in Europe by regulators.
“Internally, what I believed in and built for is now reflected back to me, wrote Eirini on LinkedIn.
“And as a business, what we knew was possible and lovingly persisted in bringing to life has now crossed the final regulatory threshold.”
100% effective with perfect use
The Inne Minilab is a non-invasive device that tracks progesterone levels through a drop of saliva.
Results, although early, have been positive so far: A small clinical study involving 300 women and more than 1,500 menstrual cycles found it to be 100% effective as a contraceptive with perfect use and 92% with typical use.
And the timing couldn’t be better.
In recent years, more and more women have been turning their backs on hormonal contraception - they are fed up with the side effects, moving into a wellness culture that favors natural options, and wanting to better understand their own bodies on their own terms.
Just this week, a new Drugwatch.com survey found that 1 in 4 women have had to switch their birth control due to life-disrupting side effects.
In entrepreneurship, ideas can sometimes be too early.
But how exciting to witness a vision, nearly a decade in the making, land with exactly the right timing.
💰 Funding, deals and investment news
📌 U.S: Visby Medical raises $55m to launch at-home STI testing to women across the US. Helping to overcome barriers such as stigma and clinic delays, Visby’s newly-authorised at-home PCR test for STIs is said to be the first single-use, disposable diagnostic of its kind. It delivers lab-grade results in under 30 minutes via smartphone. This financing round - which Visby says could rise further to $65m - was led by Catalio Capital Management. The test will launch in July. (Continue reading: FutureFemHealth)
📌 U.S: Materna Medical rebrands childbirth device and adds new investors. New investors GLIN Impact Capital, Wealthing VC Club, and Citrine Angels join Materna’s ongoing $20M Series B2 round, backing its broader push in pelvic health — a space it estimates at worth $25 billion. Materna Medical has also announced a new name for its device Materna Prep, now named Ellora, which aims to reduce pelvic floor injury during vaginal delivery. The device is currently in a major U.S trial across 20+ hospitals and is expected to go for FDA De Novo submission later this year. (Continue reading: FutureFemHealth)
🌟 Industry news from this week
📌 GLOBAL: Maven Clinic and ŌURA partner to deliver more connected experience for women's health. Maven members can now sync Oura ring data with the platform, enabling more personalized care and earlier issue detection. From managing gestational diabetes with nutrition coaching to spotting perimenopause signs through sleep and cycle tracking, the partnership aims to deliver expert support exactly when it’s needed. It follows a a recent Maven survey which found 73% of its member respondents said they track their health regularly, and 45% review their data daily. (Continue reading: FutureFemHealth)
📌 CANADA: June Health launches in Canada to tackle the midlife women's health gap at work. Aimed squarely at the “silent productivity and retention crisis” impacting employers, June Health offers end-to-end clinical support for menopause and perimenopause, mental health care, pharmacy access, and benefits navigation via a single platform. CEO Lori Casselman highlights the economic toll of untreated symptoms, estimated at $3.5B annually in Canada, while Medical Director Dr. Romy Nitsch calls for midlife health to be treated as a complex medical phase, not a lifestyle issue. The service is now available to employers and health plans nationwide. (Continue reading: FutureFemHealth)
📌 U.S: Progyny adds pelvic floor therapy to its health employer benefits platform. An estimated one in three women experience pelvic floor disorders, with one in five requiring surgery to address them. But while pelvic health is commonly linked to postpartum care, conditions extend far beyond birth recovery, affecting women from early adulthood through menopause and beyond. Progyny is to partner with digital health providers Origin and Hinge Health for this initiative, so that its members can access both in-person and virtual specialist care. (Continue reading: FutureFemHealth)
📌 U.S: Almara Women’s Health launches with physician-owned, whole-care model. An interesting alternative to VC-backed or corporate entities, Almara’s physician-owners say their model allows for greater freedom and patient-centred decision-making. The launch brings together 10 clinics in Minnesota initially, with plans for further expansion. They offer relationship-based care across the full span of women’s lives - from adolescence to menopause, fertility to mental health. “We created Almara because the healthcare system too often flattens what it means to be a woman,” said president Dr Suzin Cho. (Continue reading: FutureFemHealth)
📌 U.S/UK:'We'll invest in creating our own': US women's health brand Frida takes censorship fight to the UK. Frida, the wellness brand known for its frank approach to women’s health, has brought its Frida Uncensored platform across the Atlantic – providing “real, unfiltered resources” across life stages, from puberty to postpartum. Highlighting the ongoing online censorship of women’s health content, Frida is inviting women to contribute their stories to the movement. (Continue reading: FutureFemHealth)
📄 Govt & policy news
📌 U.S: New bill would require menopause education from health care providers. In an effort to combat misinformation, Wisconsin lawmakers are circulating a bipartisan bill in the state Capitol that would require health care providers to offer more education on menopause and perimenopause. Under the bill, the state Department of Health Services would work with health care providers to create informational materials to educate patients and reduce stigma. (Continue reading: wpr.org)
📌 UK: Women who don’t come forward for cervical cancer screening to be offered home tests. The Department of Health has said participation in cervical cancer screening currently sits at just 68.8% - well below the NHS England target of 80%. A new initiative will offer self-sample kits to test for the presence of human papillomavirus (HPV) - a group of viruses which can lead to cervical cancer. (Continue reading: Sky News)
📆 Save the date
100 female founders: HealthTech Innovators meetup at Barclays: London, 22 July, 8am- 11am
Join us on a mission to get 100 female founders and women’s health innovators in one room before the summer holidays!
FutureFemHealth is excited to be a collaborator on this meetup hosted by Barclays Innovation Hub in collaboration with the brilliant team at Nexus as well as WEALTH, Hotbed, and NXGN.
🔥 Find out more and reserve your space here. (Hurry, spaces are booking fast!) Look forward to seeing you there!
That’s all for this week! See you next time. If you’ve missed any previous newsletter issues catch them all at futurefemhealth.com and do make sure to follow us on LinkedIn.